Sputnik V Vaccine – Is It The Beginning Of The End? Or Is It Worse?
When Russia broke the news of the world’s first-ever COVID-19 vaccine, it was more than a silver lining on a cloudy day. With several myths and claims going around, this article provides an in-depth discussion about Sputnik and whether it is the right cure.
On Nov 13, 2020 – 16 minutes read
A global “sputnik moment” broke out on the 11th of August with an announcement the whole world has been eagerly waiting for. With 165 vaccines in early development and 5 vaccines being tested in Phase 3, a potential vaccine was on the verge of success. Or so we hoped.
As no further COVID-19 vaccine progress came into notice, the world began to live with the accustomed “new normal” coronavirus forced upon us. Then came the alarmingly surprising announcement of Sputnik V, a locally developed vaccine from Russia against the global pandemic COVID-19. While the whole world is on the lookout for the best medicine to overpower the deadly Corona Virus, let’s look in detail at how promising is the newly introduced vaccine!
Sputnik V Vaccine – Is It The Beginning Of The End?
In declaring the approval of their Sputnik V vaccine as the “first registered vaccine against COVID-19”, Russian President Vladimir Putin probably put the worldwide vaccine race to a surprising halt. More than hundreds of vaccines are currently developing in different parts of the world with CanSino Biologics in China and Oxford University and AstraZeneca in Britain in Phase 3.
Two months of a clinical trial is evidently too early for a vaccine to be approved. A Moscow based institute named Gamaleya Research Institute developed the formula for the said experimental coronavirus vaccine. Scroll down to know more about the vaccine.
Why does the Name “Sputnik V” ring a bell?
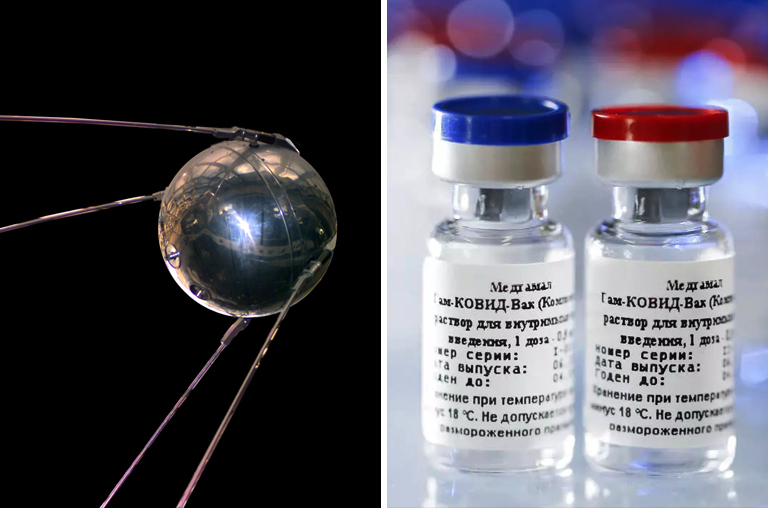
Yes, it is a familiar name not just to the Russians but to the whole world. The newly developed vaccine “Sputnik V” is nicknamed after the Russian space satellite “Sputnik” that beat the world into orbit on October 4, 1957, by the Soviet Union.
The name itself, in reference to the last century’s Cold War space race that reinvigorated space research in the world, signifies the country’s success in being the first to have a vaccine approved. Moreover, they put out a video referencing the namesake on the official website of Sputnik V.
How is the vaccine developed?
Vaccines are developed by taking viruses and weakening them so that they cannot replicate or reproduce themselves or so that they cannot replicate at all in addition to developing immunity. The main types of vaccines include virus-based, viral vector-based, protein-based, and nucleic acid-based vaccines. Sputnik V vaccine has been developed using a viral vector-based vaccine. Scientists from the Gamaleya Center have been working on adenoviral vector-based vaccines since the 1980s. As it turns out, they have become the world’s leaders in developing these types of vaccines for years.
Russian scientists used adenoviral vectors for the Sputnik V vaccine. Notedly some other COVID-19 vaccine candidates including CanSino Biologics in China and Oxford University and AstraZeneca in Britain also have been using adenoviral vectors as their base for their vaccine development. What did set Russia apart from them is the use of the two-vector platform the Gamaleya Center has developed. Now, let me break down what each of these terms is and how the formula is developed.

Adenoviruses are medium-sized, nonenveloped, common viruses that cause a range of illnesses such as common cold, sore throat, fever, bronchitis, pneumonia, and pink eye. They are found in normally transmitting common cold and adenoids.
Adenoids are a patch of tissue that is high up in the throat and is a part of the lymphatic system along with the tonsils. The lymphatic system clears away infection and helps in keeping the body fluids in balance. They work by trapping germs coming in through the mouth and nose.
Vectors are viruses, more commonly known as “vehicles or carriers” in the medical field, which have had the gene responsible for reproduction removed. For this reason, they no longer pose an infection threat to the body. Scientists around the world use these vectors to transport genetic material from a different virus, which is the virus being vaccinated against, into a human cell. In Sputnik V, they have used adenovirus as the vector.
Are Adenoviral Vectors Safe?
The use of human adenoviruses as vectors is considered extremely safe because apparently these viruses are not novel and have been around for thousands of years. As they only cause the common cold, they are said to be easiest to engineer too. They have become very popular as vectors with a lot of research available from different sources. These are further modified to use in the formula of the vaccine as to not cause illness.
How do Adenoviral Vector-based Vaccines work?
During the Sputnik V vaccine development process, a genetic material with the code of a protein (s protein) from a spike of coronavirus is inserted into an adenoviral vector. Adenovirus as vectors can induce a genetic material from another virus (here, coronavirus) into a cell. The gene from adenovirus, which causes infection, is removed. Instead, a gene with the code of a protein from coronavirus’ spike is inserted there.
The inserted element is said to be safe for the human body. It can still help the immune system of the body to react and produce antibodies to protect the body from the infection. Gamaleya Center claims that the technological platform of adenovirus-based vectors makes it faster and easier to create new vaccines.
How? By modifying the initial carrier vector (adenovirus) with genetic material from emerging viruses (coronavirus) that help to create new vaccines in a relatively short time. By the same token, such vaccines provoke a strong response from a human immune system.
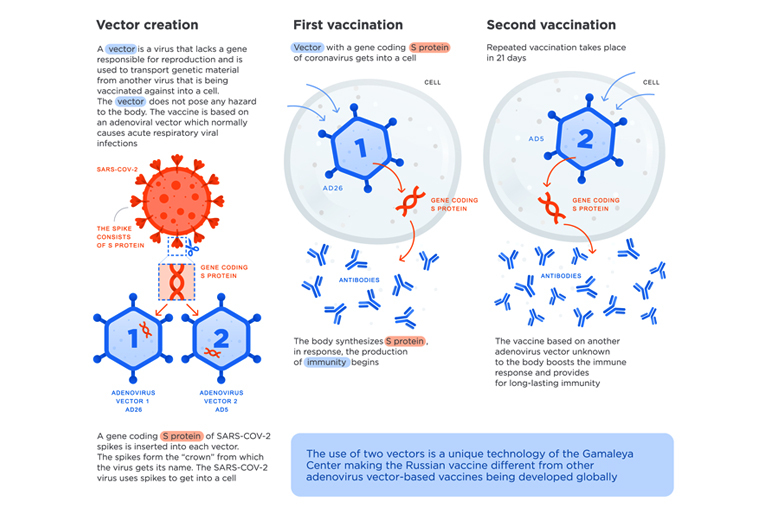
To be more precise, after the start of the COVID-19 pandemic, Russian researchers extracted a fragment of genetic material from novel coronavirus SARS-COV-2, which codes information about the structure of the spike S-protein, which forms the virus “crown” and is responsible for connection with human cells and is a structure that plugs into cells to trigger infection.
They inserted it into a familiar adenovirus vector for delivery into human cells which the immune system should recognize and use to target the pathogen should the body ever become exposed. In order to ensure lasting immunity, Russian scientists came up with an outstanding idea to use two different types of adenovirus vectors (rAd26 and rAd5) instead of one for both the first and second vaccination, which in turn is supposed to boost the effectiveness of the vaccine(1). The use of two strains of adenovirus as its base has set the Sputnik V vaccine apart from other vaccine formulas.
What are the claims?
According to Russian researchers, the vaccine produced strong antibody and cellular immune response in vaccinated bodies. Not only have they reportedly been precisely tested for antibodies in the blood serum of volunteers but also analyzed for antibodies that neutralize the coronavirus. In response to the spike S-protein of the coronavirus, they assured the ability of the immune cells of the volunteers to activate. As a result, this test has indicated the formation of both antibody and cellular immune vaccine response which in turn confirmed the high efficacy of the vaccine.
Before the start of clinical trials, the Sputnik V vaccine had gone through all stages of pre-clinical trials and experimented on different types of animals, including 2 types of primates. Phase 1 (included 76 humans) and Phase 2 trials of the vaccine were performed on about 200 humans and all the volunteers are feeling well with no unforeseen or unwanted side effects observed. Surprisingly, the Gamaleya National Research Institute of Epidemiology and Microbiology successfully developed and registered an adenoviral vector-based vaccine against Ebola, too.
Russia also mentioned that the world is lining up for the Sputnik V vaccine, about two dozens of countries and hundreds of companies. The Philippines President Rodrigo Duterte volunteered himself to be vaccinated. Sputnik V was officially registered with a certificate from the Russian Ministry of Health on August 11. Under emergency rules adopted from the COVID-19 pandemic, the Russian population can be vaccinated with the Sputnik V.
Sputnik V – A Russian Roulette in Question?
The announcement of an approved vaccine is indeed relieving. Unfortunately, the comforting news came with several unanswered questions coupled with a whole load of skepticism. Without important data shared with the world, the public fears this rushed move as a gamble in the name of power (the name itself suggests that) which has all the possibilities of a threatening backfire.
As long as the myriad questions about the safety and effectiveness of the vaccine remain unproven to the world, anyone can choose not to overlook the accusation of vaccine nationalism behind the Sputnik V. However, Russia calls international concern over the Sputnik V as “absolutely groundless” and reasoned the criticism as mere competitiveness of foreign countries.
President Vladimir Putin even mentioned that one of his daughters was given the vaccine shot and she is well now. He tried to make people realize the confidence he had in the effectiveness of the vaccine by giving a jab to his own daughter, which in turn made countless people put their trust in the vaccine.
However, not everyone took this piece of surprise as a good decision from the President himself. The vaccine has been given to Kirill Dmitriev’s (chief executive of the Russian Direct Investment Fund (RDIF)) parents who are in their seventies which has raised eyebrows in both surprise and concern. Most people have reportedly said “using a vaccine that has not been tested in a Phase 3 trial on healthy people is not science.
That is audacity.
” Similarly, Roger Lord, a research fellow at the Prince Charles Hospital likened Putin’s attempt to build trust as, “This is akin to President [Donald] Trump claiming hydroxychloroquine(2) is safe because of personal use and despite warnings from the FDA.”
Things took an interesting turn when US officials accused Russia of hacking into their vaccine research information which of course Russia vigorously denied.
Why are scientists and health experts skeptical about the Sputnik V vaccine?
Numerous global health experts from a growing list of countries raised concerns over the efficacy and safety of the said vaccine. Why? To put it bluntly, because of a lack of transparency. Because the Russian government didn’t provide any clinical data of their first two clinical trials. Most countries, including China, have been transparent in sharing data of their COVID-19 vaccine progress including their data from trails. Whereas, Russia hasn’t published any information from phase 1 or 2 to the public yet. For this reason, many have voiced their opinions like “if only Russia values transparency as much as speed” to show their disagreement.
In addition to that, Russia hasn’t cleared a large-scale clinical trial, Phase 3, yet. Although there is scientific evidence confirming the safety and efficacy of vaccines based on adenoviral vectors, Russia cannot possibly confirm whether the vaccine will work efficiently or not without going through a widespread Phase 3 trial(3). This reason alone brought widespread skepticism. They didn’t even test the required number of people to clear the first two phases.
While phase 1 and 2 early trials will provide information about how well a vaccine works, only a phase 3 trial can compare rates of infection between vaccinated and unvaccinated people and demonstrate that the vaccine does indeed prevent the infection.
“Without phase 3, they just cannot have any awareness of the potential pitfalls and determine who should get the vaccine and who shouldn’t,” said Damian Purcell, laboratory head at the Doherty Institute.
“It’s very, very risky.” The Sputnik V cleared Phase 1 and 2 trials within two months, which raised more concerns. Less than two months of human testing is “about enough time to do the first steps, a phase 1 trial that gives you some idea of immune response across more than one dose and is simply not enough time to do a reasonable efficacy workup as well, and absolutely not enough time to get any sort of reading on safety,” chemist Derek Lowe voiced his opinion.
Phase 1 and phase 2 trials are small in size and short in length of one a few months. Hence they are useful for studying common, short-term side effects such as mild fever, skin redness, or swelling, or soreness at the injection site. Short term effects like fever usually occur as a vaccine jumpstarts the immune system. This could be the case of Putin’s daughter who participated in the early trial of the Sputnik V and experienced a transient fever. Plus, some side effects may only emerge once a vaccinated person is exposed to the coronavirus in real life.
As Live Science(4) previously reported, one such side effect is known as “Antibody-Dependent Enhancement (ADE) which is a phenomenon that paradoxically leaves the body more vulnerable to infection after vaccination”. A sign of ADE, or a similar problem, would be if the people who got the vaccine in the trials actually had higher attack rates of the virus than the people who got a placebo.
Only extensive and prolonged testing can determine if a vaccine is effective and safe without any adverse side effects. To clarify, no vaccine is a hundred percent safe. It could have adverse side effects, if not tested through rigorous clinical trials. Moreover, some people, particularly those who are immunocompromised (having an impaired immune system), could be put into extreme danger. This raises more concern for the need for the phase 3 trial.
Correspondingly, one clinical trial for an HIV vaccine (which used the same virus vector as the Chinese COVID-19 trial) stimulated the rate of infections instead of providing any protection against it. What Russia is doing with the vaccine is tuning the immune response.
But if the vaccine, by any chance, tunes it the wrong way, it could accelerate the infection and those people could be carrying a terrible immune legacy through the rest of their lives as well. As there has been no comparison with a placebo, we cannot be sure of how protective the vaccine is either. The FDA in the US, to approve a vaccine, requires that a COVID-19 vaccine should at least halve the chances of a person getting infected with the virus when compared with a placebo, or inert injection.
The lack of safety standards is another problem. Who can be vaccinated and who cannot be? People with which disease shouldn’t be vaccinated? Can pregnant women, children, people with protein imbalance (who could die due to protein allergy) be vaccinated with Sputnik V? All these really important questions remain unanswered.
WHO (World Health Organization) still considers the Russian vaccine in the early experimental stage. “We don’t have sufficient information at this point to make a judgment” on the Russian vaccine, said(5) Dr. Bruce Aylward, a senior adviser to WHO’s director-general.
“We’re currently in conversation with Russia to get additional information to understand the status of that product, the trials that have been undertaken, and then what the next steps might be.”
The potential harms, according to Dr. Isaac Bogoch, an Infectious Disease Specialist, are:
- The safety of people: as we are not quite clear about the common and uncommon side effects of the vaccine.
- We have no real understanding of the benefit of the vaccine. We are not even sure if the vaccine does works.
- The cost of trust in public health as this vaccine if proved to be ineffective could create a ripple effect well beyond COVID-19.
Why is Phase 3 important?
A vaccine cannot be simply developed and rolled out. There are hurdles it must clear before it is proven to be promising. As it is extremely dangerous to be administered without clearing all phases, every vaccine goes through these phases below to be claimed as a potential vaccine for a disease. In this case, the Sputnik V vaccine hasn’t cleared phase 3 yet and hence the rise of concern over the “hurriedly developed” vaccine.
- Preclinical trial: This is the trial for testing the vaccine in animals. Through this phase, the study should answer a few questions such as “does the vaccine produce antibodies?” and “does it protect against illness?”.
- Phase 1: This is the first testing in humans. A small number of healthy humans (20-100) are tested to determine whether the said vaccine is safe or not. About 70%(6) of drugs move to the next phase.
- Phase 2: This is the testing to make sure whether the vaccine truly works. More humans (several hundred) should be tested in this phase. About 33% of drugs that make it to phase 2 trials move to the next phase.
- Phase 3: This is the most important phase in approving a vaccine. A larger number of humans (tens of thousands of humans to be specific) should be tested in this phase to confirm the effectiveness of the vaccine. Phase 3 determines the safety and efficacy of vaccines produced for any When a vaccine is cleared of this phase, it will be approved to roll out to the public. About 25% to 30% of drugs that make it to phase 3 trials move to the next stage.
- Phase 4: After the vaccine has been rolled out, phase 4 starts. It is ongoing monitoring to make sure that the vaccine is safe and doesn’t have any long-term adverse side effects to it.
Russia has decided to give the first shot to the scientists working on the vaccine, healthcare workers, teachers, and every other frontline worker(7). Basically a couple of thousands of high-risk people (people who are more prone to get infected). If the vaccine is proven to be effective beyond their plain words, this could be a brilliant idea.
However, several health experts disagree with this move alone. Instead of testing Phase 3 on frontline workers, Russia should give the vaccine to people from different age groups, demographics, and especially for people from areas where the virus is rapidly circulating. In that case, determining the effectiveness could be studied easily by comparing those who are vaccinated with those who are not. In addition to that, that particular study will provide a significant report on the age and various demographics, too.
Phase 3 clinical trial involving more than 2000 people in Russia, some people from the UAE, Saudi Arabic, Brazil, and Mexico is said to start in the coming days. They are doing Phase 3 with people around the world! Honestly, we could even say Russia didn’t do due diligence and is rushing through the process without following appropriate protocols.
The world has every right to be skeptical given that phase 1 and 2 data have not been presented yet. Health experts further accused Russia by saying “this is merely based upon their words and this is not typically the way we reach into scientific conclusions.” The COVID-19 vaccine latest update from Russia is a brilliant theory; the use of two adenoviral vectors. However, with no provided data, it remains just a brilliant theory with potential harm.
COVID-19 Vaccine: Latest Update
The mass production of the Sputnik V vaccine is expected to start in September 2020 and the mass distribution of the vaccine will start in October. At least 20 countries had expressed interest in obtaining the Sputnik V stock, including the UAE, Saudi Arabia, Indonesia, the Philippines, Brazil, Mexico, and India. So far, Russia has not introduced the Sputnik V vaccine price outside the nation(8).
All things considered, it is not even about Russia having the capacity to develop one effective vaccine this quickly. As a matter of fact, there is tremendous capacity in the country with brilliant scientists along with the infrastructure to do so. It is about the lack of phase 3 trials before integrating the vaccine into the community vaccination process. We have to remember that this potential vaccine will be given to healthy people which makes all the difference if the vaccine is not proven effective later.
It is increasingly becoming difficult to open an optimistic eye to the world around us. With all that is going around lately, no bad news is a surprise anymore. The lack of required information on the table gives us every right to believe that this could turn the other way round too. If that does happen, Russia’s rushed declaration could impact the world on a whole new level beyond COVID-19’s strike itself.
To speak with an optimistic eye, if this cure is the COVID-19 vaccine progress we have waited for with crossed fingers, it will be the victory of humankind in every sense. Let us all hope Russia and every other country that is working on the COVID-19 vaccine is carrying out all the precautions that are required to produce the vaccine. Let us hope we can pull this off, together like how it is supposed to be.

Subscribe to Newsletter
Elevate your routine, stay on trend, and embrace a personalized beauty journey with our curated insights.






Write a Comment